NIH Awards $7.5 Million to Ankur Singh for Pioneering Human Immune Organoid Research

Bioengineer Ankur Singh works to create functional models of the human immune system in the lab. (Credit: Ankur Singh)
The National Institutes of Health (NIH) has awarded $7.5 million to Ankur Singh, Carl Ring Family Professor in the George W. Woodruff School of Mechanical Engineering (ME) and professor in the Wallace H. Coulter Department of Biomedical Engineering (BME) at Georgia Tech and Emory, for his pioneering research in creating functional models of the human immune system in the lab.
The funding, sourced from the National Institute of Allergy and Infectious Diseases, supports two projects aimed at developing human immune organoids, which are sophisticated models engineered to replicate and study the natural human immune responses. The research could revolutionize vaccine development and immune system research, particularly for aging populations.
"Little advancement has been made in this area due to the complex nature of the immune system and the challenges of making a functional human immune tissue outside the body,” said Singh, who is also director of the Center for Immunoengineering at Georgia Tech. “I am grateful to the NIH for supporting our work, which will enable us to develop an advanced technology that can help solve the problems of emerging infections and enhance our timely response to them.”
Building Next-Generation Human Immune Organoids
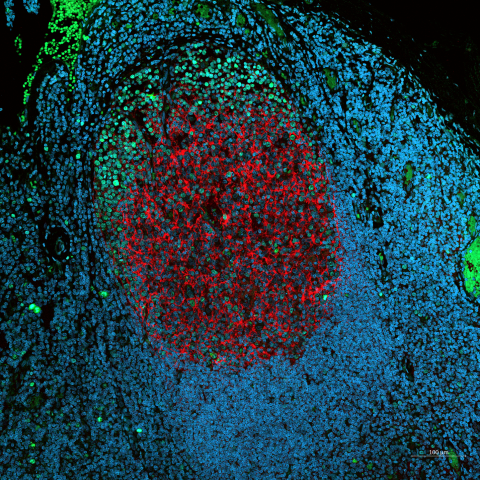
The goal of Singh’s first project is to replicate the complex environment of germinal centers (GCs) — the sites within lymph nodes where B cells are trained to produce the antibodies crucial for fighting infections. While animal models and current engineered systems have offered insights, they fall short in recreating the intricate processes that occur in human GCs, which limits their utility in vaccine development and understanding immune responses.
Singh’s method involves using a hydrated polymer-based gel material to create a structure that mimics the environment of lymphoid tissue in the body. By adding human immune cells (like B cells, T cells, and support cells) into this gel, the project tries to recreate how B cells mature into specialized immune cells that are important for a strong and lasting immune response. This advancement will allow scientists to grow and study these cells in the lab and use them for better vaccine testing, therapeutic development including cell-based therapies, and to deepen our understanding of the immune system.
The second project addresses a pressing issue in public health: the decline in immune function with age. As people age, their ability to mount effective immune responses against new infections diminishes, leading to higher mortality rates from diseases such as influenza and Covid-19. However, the underlying mechanisms — whether due to defects in aged B cells, impaired T cells, or changes in the lymphoid tissue environment — remain poorly understood.
Singh’s research proposes the development of an “aged B cell follicle” organoid, a novel platform that replicates the lymphoid microenvironment of older individuals. This system will allow researchers to dissect the factors driving age-related declines in immune function, offering a new tool for studying how aged B cells respond to antigens and identifying molecular targets to rejuvenate immune responses.
A Pioneering Step Forward in Immunology Research
The broader impact of Singh’s organoid research is wide-ranging. By enabling the study of human immune responses in a controlled, reproducible environment, the organoids could dramatically accelerate the development of vaccines and immunotherapies. The models could also provide new insights into whether a particular vaccine will be effective for a given individual, potentially reducing the time and cost of clinical trials.
Singh’s aged immune organoid platform could serve as a rapid screening tool for identifying older individuals who are likely to respond poorly to vaccines, enabling more personalized and effective vaccination strategies for that population. The models could be particularly useful in the context of pandemics or seasonal flu outbreaks, where timely and effective immunization is critical.
“By securing this substantial NIH funding, Singh’s work is poised to make a significant impact on both the scientific community and public health,” said Andrés García, executive director of the Parker H. Petit Institute for Bioengineering and Bioscience, Regents' Professor in ME, the Petit Director's Chair in Bioengineering and Bioscience, and a collaborator on Singh’s first project. “This innovative immunoengineering research not only promises to advance our understanding of immune system function and aging, but also holds the potential to transform vaccine development, offering new hope for more effective disease prevention strategies across the lifespan.”
The NIH’s investment in Singh’s research underscores a growing recognition of the need for innovative approaches to studying human immunity. The Food and Drug Administration Modernization Act 2.0, for example, promotes the use of organs-on-chip technologies in the service of drug development. As organoid technologies continue to evolve, they could come to represent the future of immunological research, providing powerful new tools to combat infectious diseases and improve health outcomes globally.
"Reflecting on the pandemic, we relied on years of research to develop vaccines and understand immune responses,” Singh said. “This new technology will allow us to innovate more rapidly and take bold steps toward creating an immune system outside the body.”
---
Key collaborators on the first project include Andrés García; Ahmet Coskun, the Bernie-Marcus Early-Career Professor in BME; and Dr. Ignacio Sanz, Mason I. Lowance Professor of Medicine and Pediatrics and chief of the chief of the Division of Rheumatology at Emory School of Medicine.
Key collaborators on the second project include Coskun; Jeremy Boss, professor and chair of the Department of Microbiology and Immunology at Emory School of Medicine; and Ranjan Sen, senior investigator in the Laboratory of Molecular Biology and Immunology at NIH’s National Institute on Aging.